

Reactivity Series can help to predict the outcome of the reaction between some metals and certain acids. NCERT Solutions For: Class 10 Science Chapter 3 Metals and Non-metals Reaction of Calcium with Cold Water: Calcium readily reacts with cold water to form calcium hydroxide and hydrogen gas.Ĭa(s) + 2H 2O(l) → Ca(OH) 2 (aq) + H 2(g).Reaction of Potassium with Cold Water: Potassium reacts with cold water to produce potassium hydroxide and hydrogen gas.

Metals from potassium to calcium react with cold water to form their respective hydroxides and liberate hydrogen gas. The reactivity series is used to anticipate the results of the reaction between metals and water. Read More: CBSE Class 10 Metals and Non-Metals Important Question
It is instrumental in anticipating the outcome of the reactions between metals and certain acids. It is used to acquire information on the reactivity of the metals toward water and acids. It is used to predict whether a metal can displace another metal in a single displacement reaction (a reaction in which one element is displaced by another element in a compound). It allows us to ascertain how the metal will react. The reactivity series is useful in several ways: H + (Non-Metal, Reference for Comparison) It must be noted that the metals in Red react with cold water, those in Orange cannot react with cold water but can react with acids, and those in Blue can only react with some strong oxidizing acids. The reactivities of metals in the descending order is given below along with their corresponding ions. The metals situated above hydrogen are more reactive than hydrogen and have the potential to displace hydrogen from water or acids to liberate H 2 gas.Īlso Read: Difference Between Rust and Corrosion. To isolate the metals from their ores and other compounds, a large amount of energy is consumed for metals at the top of the series. In addition to electro positivity, the electron-donating ability of the metal also reduces while travelling down the series. Hence, sodium is more electropositive than lithium but less electropositive than potassium. The electropositive nature of metals decreases as we move down the series. The reactivity of Metals chart gives a detailed understanding of the reactivity of different metals with different elements. A metal situated above in the reactivity series (more reactive) can displace the metal placed below it (less reactive) from its compound. For example, Zinc can displace copper from the copper sulfate solution as it is placed above copper and therefore, is more reactive. Hence, the upper part of the series contains powerful reducing agents as they are easily oxidized, and corrode easily. The reducing power of the metals decreases as we move from the top of the table to its bottom.  The top of the reactivity series comprises the most reactive elements, which are not available in nature in the free state and the bottom of the series includes the least reactive elements.
The top of the reactivity series comprises the most reactive elements, which are not available in nature in the free state and the bottom of the series includes the least reactive elements. 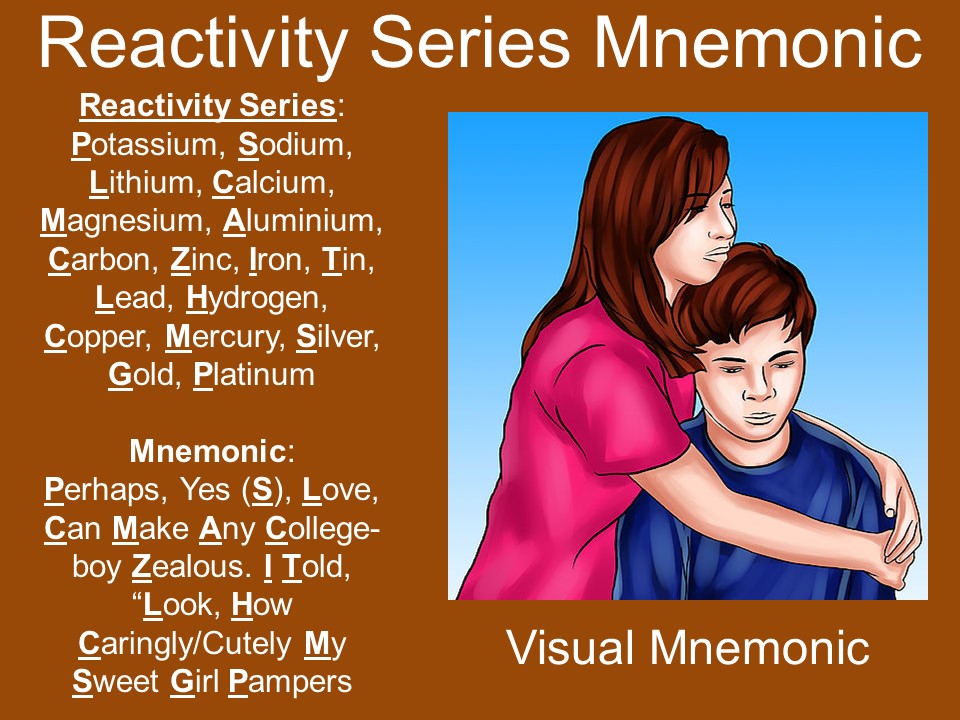
Here are the salient features of the reactivity series: Salient Features of the Reactivity Series However, metals from Zinc to Mercury can be easily extracted by reducing their oxides, which is comparatively cheaper. Electrolysis is used to extract metals that are placed higher in the reactivity series.On the other hand elements such as magnesium, iron, and aluminium react with steam to form oxides and hydrogen gas. Due to their high reactivity, Potassium, Sodium, Lithium, and, Calcium reacts with cold water to form hydroxides and hydrogen gas.But, different metals have different reactivities toward oxygen such as gold, which is an unreactive metal and doesn’t readily form oxides when exposed to air. Most of the metals react with oxygen to form metal oxides.Higher ranking metals generally show a more metallic nature than that placed lower in the series.The higher the metal placed in the series, the more reactive it is and the more strongly it reacts with water, oxygen, and acid.Reactivity series can be used to predict if a metal can displace another in a single displacement reaction.Reactivity series of metals, also referred to as the activity series, is the arrangement of metals in the descending order of their activities.








 0 kommentar(er)
0 kommentar(er)
